You know that feeling you get when you pour a glass of tap water? It seemed like it was missing that wonderful sparkle? It turns out that the tiny, invisible gas known as carbon dioxide (CO2) may be connected to the missing magic.
When it comes to the CO2 naturally found in water, it adds a refreshing tang and brings it to life, enhancing its taste. However, there’s more to this small gas than just its flavor. It has a significant impact on the health and safety of our tap water, which is quite surprising.
So, what effect does carbon dioxide have on tap water? Does it impact the flavor? Could it have an impact on the environment? And what relevance does all this have to you and your daily glass of water?
Understanding CO2 in Water: An Engaging Conversation
Welcome to water’s intriguing CO2 world! If you drink water every day, this chemical can change the taste, the acidity, and even the way it affects the environment.
What makes this issue so exciting? Let’s investigate it further and see what we can find:
The Mystery of the Fizz
Let’s start by exploring the natural sources of CO2 in water. Water is enriched with the invisible gas through various natural processes, such as raindrops absorbing atmospheric CO2 and groundwater interacting with rocks and minerals. However, the real transformation occurs when CO2 dissolves and becomes carbonic acid. This process results in the well-known tangy flavor and causes a slight decrease in the pH of the water, resulting in increased acidity.
Temperature and Pressure: The Fizz Controllers
It’s interesting to note that colder water has the ability to hold more CO2. That’s why your morning glass can sometimes have a fizzy texture, while a warm bottle allows those bubbles to escape, resulting in a flat beverage. Understanding the behavior of CO2 in water hinges on grasping the connection between temperature and pressure. When pressure decreases (similar to opening a bottle), the dissolved CO2 is released, resulting in the water losing its carbonation. On the other hand, when pressure is increased (similar to carbonating water), more CO2 is forced to dissolve, resulting in that delightful fizz.
Exploring the World of Water: The Effects of Carbon Dioxide
Although the carbonation adds a delightful fizz, the impact of CO2 extends far beyond just enhancing the taste. The impact it has on acidity can potentially affect one’s health. Although research indicates that healthy teeth are generally unaffected, individuals with pre-existing dental issues should take their water choice into consideration.
In addition, there is some research indicating that the carbonic acid found in sparkling water could potentially assist with digestion and the absorption of nutrients. However, further research is required to fully understand this potential benefit.
Exploring the Intricacies of Plumbing and its Subtle Impact:
CO2’s acidic nature can be a concern for homeowners. Similar to a water scientist, it is worth noting that highly acidic water has the potential to corrode pipes and increase metal content in the water, although this impact is generally minimal on modern plumbing systems. Having a good grasp of your local water source and its CO2 levels can enable you to make well-informed choices regarding your plumbing system.
We are only scratching the surface of our exploration! Be sure to keep an eye out for upcoming segments of the discussion, where we’ll explore the fascinating realm of artificially carbonated water, its environmental implications, and helpful advice for making conscious decisions about your water usage.
Important Effects of CO2 on Tap Water: Immerse yourself in the world of carbonation!
Have you ever wondered why your tap water occasionally has small bubbles? The effervescent sensation is a result of carbon dioxide (CO2), a naturally present gas that has intriguing impacts on our everyday hydration.
Let’s explore the fascinating science behind CO2 in tap water and its diverse impacts on:
1. Taste:
The most noticeable impact of CO2 is on taste. With increased levels of CO2, there is a noticeable effervescence that adds a pleasant tingle, making it a sought-after feature in carbonated water. On the other hand, a decrease in CO2 leads to a “flat” taste that may be less appealing to some. However, it’s not solely a matter of personal preference!
2. Acidity & Health:
In addition to affecting taste, the presence of CO2 in water can cause a slight decrease in pH, resulting in a slightly acidic nature. Although there are concerns about its effect on tooth enamel, research indicates that it poses little harm to healthy teeth. However, it is advisable for individuals with pre-existing dental issues to seek guidance from a dentist. It is worth noting that certain studies have indicated that the carbonic acid found in sparkling water could potentially assist with digestion and the absorption of nutrients.
3. Plumbing & Corrosion:
The acidity of water with high levels of CO2 can have an impact on plumbing systems. In regions where there are older metal pipes, the presence of acidic water may expedite the process of corrosion, which could potentially result in higher metal levels in the water. Fortunately, the majority of contemporary water treatment systems effectively address this problem. So, unless you reside in an area with documented issues, feel free to savor your refreshing tap water without any worries!
4. Environment & Sustainability:
Although sparkling water may appear to be a more enjoyable option, bottled water has a considerable impact on the environment. Plastic production, transportation, and waste are major contributors to both greenhouse gas emissions and plastic pollution. Opting for naturally carbonated tap water is a highly sustainable decision that helps minimize your carbon footprint and reduces plastic waste.
What is the role of carbon dioxide in the water?
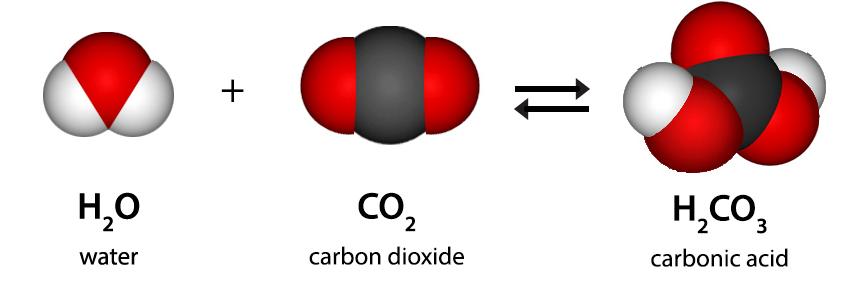
Picture a glass of water without that invigorating fizz, that revitalizing snap. Carbon dioxide (CO2), a small and invisible gas, seems to have a significant impact on your tap water, potentially explaining the absence of that special quality. Just like a water scientist, it’s fascinating to explore how the natural presence of CO2 in water can affect both its taste and health.
Is carbon dioxide responsible for water pollution?
That’s not quite accurate! Although the presence of CO2 in treated tap water is within safe limits, it is important to be aware of the potential harm that rising CO2 levels in our environment can have on aquatic life. However, gaining a deeper understanding of its influence allows us to make well-informed decisions regarding water consumption and its impact on the environment.
What is the amount of carbon dioxide present in drinking water?
The levels of CO2 in tap water can differ based on the origin and the methods used for treatment. Typically, it is found in small, regulated quantities to preserve flavor, acidity, and inhibit bacterial growth. Excessive amounts, though, can give the water an unpleasantly sour taste and potentially impact its safety.
How can carbon dioxide levels be decreased in water?
If you notice that your tap water has a fizzy or acidic taste, there are methods to regulate the levels of CO2:
When water is heated, the release of CO2 reduces its tang.
When it comes to filtration, it’s important to select filters that are specifically designed to remove excess CO2. Choosing still water has a straightforward method to mitigate any potential drawbacks of increased CO2 levels.
Understanding the presence of CO2 in your tap water allows you to make informed decisions regarding your drinking experience and its impact on the environment.
Sum-up
Wow, that was quite an immersive exploration into the captivating realm of CO2 and its influence on our cherished tap water! As we’ve observed, this minuscule gas has a more significant impact than merely providing a touch of effervescence. It has an impact on flavor, acidity, and even the well-being of our surroundings and our bodies.



Leave a Reply